Drs. Moses Joloba, David Kateete and Charles Batte win award of USD 1.75 million from the National Health Institutes, USA to support the development of research capacity for data science for infectious and Non-communicable diseases.
Classic list
News
A project funded by the Government of Uganda through Makerere University Research and
Innovations fund (Mak-RIF)
Project Outcomes
1) Capacity Building Training, Sops, Draft Guidelines, Equipment and Supplies
Makerere University has over the years pioneered training and research in different fields of national
importance. As such, a team from the school of biomedical sciences set out to establish capacity for
human tissue and organ biobanking. Staff from Makerere University School of Biomedical Sciences,
Kawempe National Referral Hospital, and Makerere University Health Services received training in
collection, processing, analysis and cryopreservation of human sperm and umbilical cord blood for
transplant. Standard operating procedures(SOPs) were developed and are being utilized. Trained staff
are now competent in the collection, processing, analysis and storage of cord blood and sperm. This
sets ground for establishing training programs to train university students and conduct research in the
collection, processing, analysis and storage of umbilical cord blood and sperm.
With this backbone, the University is taking baby steps towards establishing an organ and tissue
transplant unit. By developing capacity to train and conduct research in this field, the University will
contribute knowledge towards standardizing and improving processes involved in human tissue and
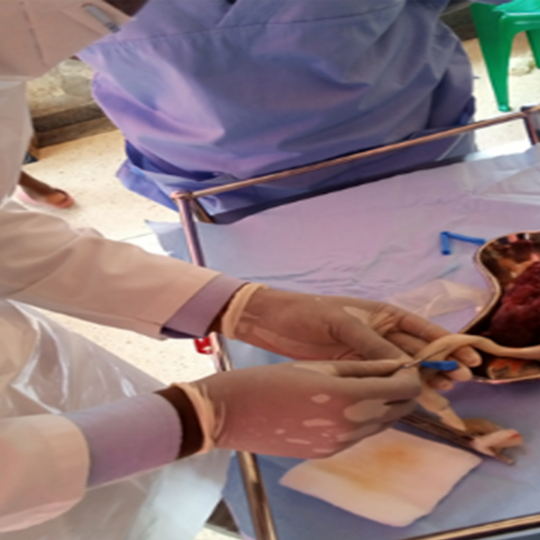
organ biobanking.2) Semen Collection and Biobanking
Semen samples from volunteers were collected, processed, analyzed and stored in liquid nitrogen at –
196 degrees. The process of sample collection involved identification of healthy volunteers/donors
who met a defined inclusion criteria. Prior to donation, each participant was taken through the informed
consent process and written consent was obtained. Clear instructions were given to the donors who
proceeded to collect samples in a designated room. Sample collection and processing followed
techniques that maximized retrieval of viable sperm. The samples were assigned unique IDs and
labelled with barcode cryo-labels. Cryopreservation involved slow -freezing prior to permanent storage
at -196 degrees in liquid nitrogen, following seminogram SOPs and WHO 2010 guidelines.
These samples are currently at -196 liquid nitrogen storage and are available to the general public who
can access them through a catalog. These samples are also available to scientists with interest of
venturing into research for the purpose of generating new knowledge in the Ugandan setting.
Page 12 of 183) Cord Blood Collection, Stem Cell Isolation and Cryopreservation
Umbilical cord blood was collected from study participants at Kawempe General Hospital following
standard operating procedures. Prior to collection, healthy mothers in their third trimester attending the
antenatal clinic were screened, consented and recruited into the study following a defined inclusion
criteria. Umbilical cord blood was collected aseptically from consented study participants during
childbirth.
Umbilical cord blood processing, volume reduction and stem cell recovery was done at the Makerere
University Immunology laboratory following the developed SOPs. The harvested stem cells were
tested for viability before cryopreservation. Stem cells processed from this blood are available at -196
Oc. Potential recipients can access these cells on the IBRH3AU catalog. These cells are also available
to researchers who wish to conduct research in stem cell biology.4) Functional Tissue and Organ Catalog
The team has developed an online Catalog that provides an unlimited access to well characterized and
annotated human sperm, umbilical cord blood (stem cells and plasma) and other Biospecimens. It is a
single convenient point of access to a pool of Biospecimens and tissues, it provides an unlimited easy
access to high quality Biospecimens, human tissues and organs by potential recipients, researchers and
the general public. Through the catalog one is able to search and request for biospecimens or human
tissues/organs for health care or basic research needs. This resource is available to the general public
at URL: www.ibru.mak.ac.ug/Catalog. The catalog will enable users to perform basic and advanced
searches, e.g. (Participant – by sex, age, ethnicity, etc., or Biospecimen type among others). From the
search results users can submit a request for access to the Makerere advisory board on organ and tissue
biobanking for consideration.

As Makerere University Biomedical Research Centre [MakBRC], we believe in science for many reasons: 1st, to sustain lives, 2nd, make living simple for people in societies where we live.
Welcome to MakBRC podium in the Science week at Kololo Airstrip, explore the importance and the future of Science with MakBRC.
Mwaka, Erisa Sabakaki, Deborah Ekusai Sebatta, Joseph Ochieng, Ian Guyton Munabi, Godfrey Bagenda, Deborah Ainembabazi, and David Kaawa-Mafigiri. “Researchers’ perspectives on return of individual genetics results to research participants: a qualitative study.” Global Bioethics 32, no. 1 (2021): 15-33.
Ochieng, Joseph, Betty Kwagala, John Barugahare, Erisa Mwaka, Deborah Ekusai-Sebatta, Joseph Ali, and Nelson K. Sewankambo. “Perspectives and ethical considerations for return of genetics and genomics research results: a qualitative study of genomics researchers in Uganda.” (2021).
Ochieng, Joseph, Betty Kwagala, and Nelson Sewankambo. “Collection and use of human materials during TB clinical research; a review of practices.” (2021).
Ali, Joseph, Betty Cohn, Erisa Mwaka, Juli M. Bollinger, Betty Kwagala, John Barugahare, Nelson K. Sewankambo, and Joseph Ochieng. “A scoping review of genetics and genomics research ethics policies and guidelines for Africa.” BMC Medical Ethics 22, no. 1 (2021): 1-15.
Mbalinda, Scovia Nalugo, Sabrina Bakeera-Kitaka, Derrick Lusota Amooti, Eleanor Namusoke Magongo, Philippa Musoke, and Dan Kabonge Kaye. “Ethical challenges of the healthcare transition to adult antiretroviral therapy (ART) clinics for adolescents and young people with HIV in Uganda.” BMC medical ethics 22, no. 1 (2021): 1-14.
Kaye, Dan Kabonge. “Motivation to participate and experiences of the informed consent process for randomized clinical trials in emergency obstetric care in Uganda.” BMC Medical Ethics 22, no. 1 (2021): 1-12.
Nichol, Ariadne A., Erisa S. Mwaka, and Valerie A. Luyckx. “Ethics in Research: Relevance for Nephrology.” In Seminars in Nephrology, vol. 41, no. 3, pp. 272-281. WB Saunders, 2021.
Kamuya, Dorcas, Mary A. Bitta, Adamu Addissie, Violet Naanyu, Andrea Palk, Erisa Mwaka, Eunice Kamaara et al. “The Africa Ethics Working Group (AEWG): a model of collaboration for psychiatric genomic research in Africa.” Wellcome Open Research 6, no. 190 (2021): 190.
Wolde, Telahun Teka, Rosemary Musesengwa, Andrea Palk, S. Mwaka Erisa, Violet Naanyu, Adamu Addissie, and Getnet Tadele. “Ethics review of multicenter neuro-psychiatric & neurodevelopmental genetics research protocols: a case study of the NeuroDev & NeuroGap-Psychosis studies.” Wellcome Open Research 6, no. 193 (2021): 193.
Kaye, Dan Kabonge. “Why ‘understanding’of research may not be necessary for ethical emergency research.” Philosophy, Ethics, and Humanities in Medicine 15, no. 1 (2020): 1-8.
Mbalinda, Scovia Nalugo, Sabrina Bakeera-Kitaka, Derrick Lusota Amooti, Eleanor Namusoke Magongo, Philippa Musoke, and Kaye K. Dan. “Ethical challenges of the healthcare transition to adult Antiretroviral therapy (ART) clinics for HIV infected adolescents and young people in Uganda.” (2020).
Barugahare, John, Fredrick Nelson Nakwagala, Erisa Mwaka Sabakaki, Joseph Ochieng, and Nelson K. Sewankambo. “Ethical and human rights considerations in public health in low and middle-income countries: an assessment using the case of Uganda’s responses to COVID-19 pandemic.” BMC Medical Ethics 21, no. 1 (2020): 1-12.
Ochieng, Joseph, Erisa Mwaka, Betty Kwagala, and Nelson Sewankambo. “Evolution of research ethics in a low resource setting: A case for Uganda.” Developing world bioethics 20, no. 1 (2020): 50-60.
Obasa, A. E., S. Singh, E. Chivunze, T. Burgess, F. Masiye, T. Mtande, J. Ochieng et al. “Comparative strategic approaches to COVID-19 in Africa: Balancing public interest with civil liberties.” South African medical journal= Suid-Afrikaanse tydskrif vir geneeskunde 110, no. 9 (2020): 858.
Barugahare, John, and Paul Kutyabami. “Nature and history of the CIOMS International Ethical Guidelines and implications for local implementation: A perspective from East Africa.” Developing world bioethics 20, no. 4 (2020): 175-183.
Newsletter